Enhanced single-molecule magnetism in dysprosium complexes of a pristine cyclobutadienyl ligand†
Abstract
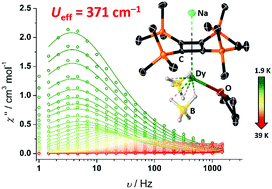
Intact transfer of the cyclobutadienyl ligand [C4(SiMe3)4]2− to yttrium and dysprosium (M) produces the half-sandwich complexes [M{η4-C4(SiMe3)4}(BH4)2(THF)]− as coordination polymers with bridging sodium or potassium ions. The dysprosium versions are single-molecule magnets (SMMs) with energy barriers of 371(7) and 357(4) cm−1, respectively. The pristine cyclobutadienyl ligands provide a strong axial crystal field that enhances the SMM properties relative to related cyclopentadienyl compounds.


 Please wait while we load your content...
Please wait while we load your content...