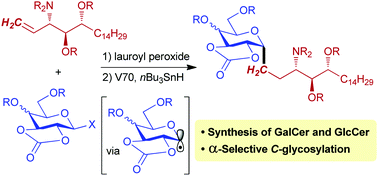
Synthesis of CH2-linked α-galactosylceramide and its glucose analogues through glycosyl radical-mediated direct C-glycosylation†
Abstract
We describe a new synthetic approach for C-linked glycolipid analogues, in which the cleavable O-glycosidic linkage is replaced by a carbon unit. Direct C-glycosylation of a conformationally constrained and stable C1-sp3 hybridized xanthate carbohydrate with carefully designed sphingosine units afforded the CH2-linked analogue of antitumor-active KRN7000 and its glucose congener.


 Please wait while we load your content...
Please wait while we load your content...