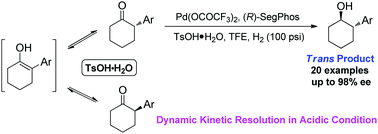
Palladium-catalyzed asymmetric hydrogenation of 2-aryl cyclic ketones for the synthesis of trans cycloalkanols through dynamic kinetic resolution under acidic conditions†
Abstract
The first efficient palladium-catalyzed asymmetric hydrogenation of 2-aryl cyclic ketones has been described through dynamic kinetic resolution under acidic conditions, providing a facile access to chiral trans cycloalkanol derivatives with excellent enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...