Palladium-catalyzed α-arylation of indolin-3-ones†
Abstract
A method for the catalytic α-arylation of indolin-3-ones was developed. The catalytic system comprising Pd(dba)2 and PAd3 was found to be optimal for the transformation. The protocol features broad functional group compatibility in that a range of arylated indoxyl derivatives bearing a fully substituted carbon center was synthesized with high efficiency. A preliminary bioassay study revealed that the selected indole-substituted indolin-3-ones exhibit favorable cytotoxic activities against HCT-116 cancer cell line.
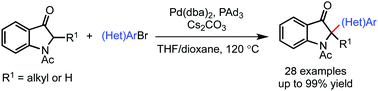


 Please wait while we load your content...
Please wait while we load your content...