A copper-catalyzed insertion of sulfur dioxide via radical coupling†
Abstract
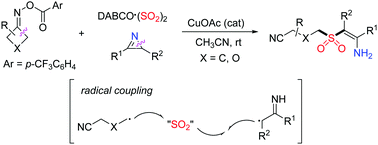
A copper-catalyzed three-component reaction of O-acyl oximes, DABCO·(SO2)2, and 2H-azirines under mild conditions has been achieved. This protocol provides an efficient route for the construction of various tetrasubstituted β-sulfonyl N-unprotected enamines in moderate to good yields with excellent stereoselectivity and regioselectivity. Notably, this method represents a rare example of 2H-azirines as useful synthons for β-functionalized N-unprotected enamines. Preliminary mechanistic studies indicate that the reaction proceeds through coupling of a sulfonyl radical and α-carbon radical via copper-catalyzed ring-opening C–C bond cleavage of O-acyl oxime and C–N bond cleavage of 2H-azirine with the insertion of sulfur dioxide.


 Please wait while we load your content...
Please wait while we load your content...