Rhodium-catalyzed Sommelet–Hauser type rearrangement of α-diazoimines: synthesis of functionalized enamides†
Abstract
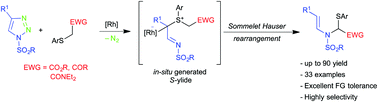
An efficient rhodium catalyzed Sommelet–Hauser type rearrangement of sulfur ylides derived from α-thioesters and N-sulfonyl-1,2,3-triazoles has been successfully accomplished for the synthesis of various functionalized enamides. The developed reaction involves the unprecedented [2,3]-sigmatropic rearrangement of sulfur ylides with the imine motif. Importantly, the method works well with various substituted α-thioesters/-amides/-ketones and substituted N-sulfonyl-1,2,3-triazoles and allows the synthesis of diverse enamide derivatives in good to excellent yields. The reaction was also successfully extended to the one-pot synthesis of enamides from terminal alkynes.


 Please wait while we load your content...
Please wait while we load your content...