Neutron diffraction structural study of CO2 binding in mixed-metal CPM-200 metal–organic frameworks†
Abstract
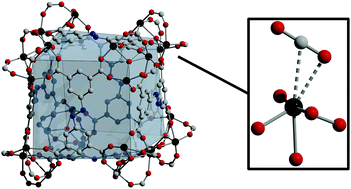
Metal–organic frameworks featuring open metal coordination sites have been widely studied for the separation of gas mixtures. For CO2/N2 separations, these materials have shown considerable promise. Herein, we report the characterization of a subset of the well-known PCN-250 class of frameworks upon CO2 adsorption via powder neutron diffraction methods. Noteably, in contrast to previously reported data, they display only moderate CO2 adsorption enthalpies, based on metal cation–CO2 interactions. Further, we show charge balance in these materials is likely achieved via ligand vacancies rather than the presence of μ3-OH groups in the trimetallic cluster that comprises them.


 Please wait while we load your content...
Please wait while we load your content...