The eukaryotic tRNA-guanine transglycosylase enzyme inserts queuine into tRNA via a sequential bi–bi mechanism†
Abstract
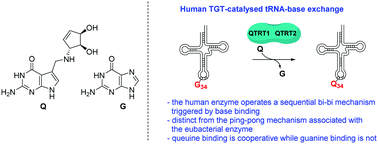
Eukaryotic tRNA-guanine transglycosylase (TGT) – an enzyme recently recognised to be of potential therapeutic importance – catalyses base-exchange of guanine for queuine at the wobble position of tRNAs associated with 4 amino acids via a distinct mechanism to that reported for its eubacterial homologue. The presence of queuine is unequivocally required as a trigger for reaction between the enzyme and tRNA and exhibits cooperativity not seen using guanine as a substrate.


 Please wait while we load your content...
Please wait while we load your content...