A bioorthogonally activatable photosensitiser for site-specific photodynamic therapy†
Abstract
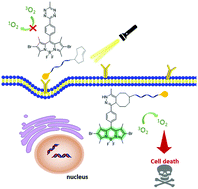
A boron dipyrromethene based photosensitiser substituted with a 1,2,4,5-tetrazine moiety has been prepared of which the photoactivity can be activated upon an inverse-electron-demand Diels–Alder reaction with trans-cyclooctene derivatives. By using a biotin-conjugated trans-cyclooctene to tag the biotin-receptor-positive HeLa cells, this photosensitiser exhibits site-specific activation through cycloaddition, leading to high photocytotoxicity.


 Please wait while we load your content...
Please wait while we load your content...