Cyclization of a G4-specific peptide enhances its stability and G-quadruplex binding affinity†
Abstract
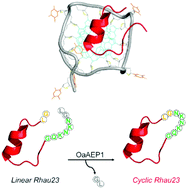
G-quadruplexes (G4) are non-canonical nucleic acid structures with important implications in biology. Based on an α-helical fragment of the RHAU helicase that displays high specificity for parallel-stranded G-quadrplexes, herein we demonstrate its head-to-tail cyclization by a high-efficiency ligase. The cyclic peptide exhibits superior stability and binding affinity to a G-quadruplex, and can serve as an excellent investigational tool for chemical biology applications.


 Please wait while we load your content...
Please wait while we load your content...