Paper-based pump-free magnetophoresis†
Abstract
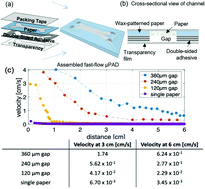
Microfluidic magnetophoresis is a powerful technique that is used to separate and/or isolate cells of interest from complex matrices for analysis. However, mechanical pumps are required to drive flow, limiting portability and making translation to point-of-care (POC) settings difficult. Microfluidic paper-based analytical devices (μPADs) offer an alternative to traditional microfluidic devices that do not require external pumps to generate flow. However, μPADs are not typically used for particle analysis because most particles become trapped in the porous fiber network. Here we report the ability of newly developed fast-flow microfluidic paper-based analytical devices (ffPADs) to perform magnetophoresis. ffPADs use capillary action in a gap between stacked layers of paper and transparency sheets to drive flow at higher velocities than traditional μPADs. The multi-layer ffPADs allow particles and cells to move through the gap without being trapped in the paper layers. We first demonstrate that ffPADs enable magnetic particle separations in a μPAD with a neodymium permanent magnet and study key factors that affect performance. To demonstrate utility, E. coli was used as a model analyte and was isolated from human urine before detection with a fluorescently labeled antibody. A capture efficiency of 61.5% was then obtained of E. coli labeled magnetic beads in human urine. Future studies will look at the improvement of the capture efficiency and to make this assay completely off-chip without the need of a fluorescent label. The assay and device described here demonstrate the first example of magnetophoresis in a paper based, pump free microfluidic device.


 Please wait while we load your content...
Please wait while we load your content...