Colorimetric detection of Cr3+ ions in aqueous solution using poly(γ-glutamic acid)-stabilized gold nanoparticles†
Abstract
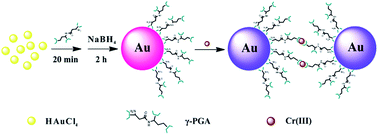
Detection of heavy metal ions in water is of paramount significance for environmental pollution control. Here, we report the use of γ-polyglutamic acid (γ-PGA)-stabilized gold nanoparticles (γ-PGA-Au NPs) as a probe to sense trivalent chromium (Cr3+) in aqueous solution. In our study, γ-PGA-Au NPs were first formed through one-step sodium borohydride reduction of Au salt in the presence of γ-PGA. The formed γ-PGA-Au NPs with a mean particle size of 4.6 nm show desirable colloidal stability and a significant color change from wine red to gray after exposure to Cr3+ ions, which is visible to the naked eye and easily detected by colorimetric assay using UV-vis spectrometry. The limit of detection of Cr3+ ions is 100 ppb by the naked eye and 0.2 ppb by UV-vis spectroscopy, respectively. We further show that the detection of Cr3+ using γ-PGA-Au NPs has excellent selectivity, and the recovery percentage is higher than 82% for different water samples such as lake water, river water, tap water or mineral water. Our study demonstrates that γ-PGA-Au NPs can be utilized as an efficient probe for colorimetric sensing of Cr3+ ions in a water environment.


 Please wait while we load your content...
Please wait while we load your content...