FTIR based kinetic characterisation of an acid-catalysed esterification of 3-methylphthalic anhydride and 2-ethylhexanol
Abstract
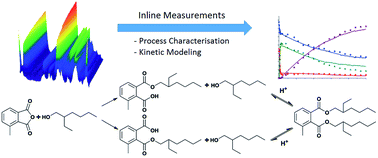
In this study, an inline analytical method was designed and applied in process characterisation and development. The model reaction is the two-step diesterification of 3-methylphthalic anhydride with 2-ethylhexanol consisting of alcoholysis as the first step, followed by an acid-catalysed, second esterification step leading to the corresponding diester. The final product is a potential, alternative plasticiser. For the inline measurements, attenuated total reflection Fourier transformed infrared spectroscopy (ATR-FTIR) was implemented. In order to evaluate the spectra recorded during the reaction, a chemometric model was established. In this work, Indirect Hard Modeling (IHM), a non-linear modeling approach was employed. The respective model was calibrated by using offline samples analysed with gas (GC) and liquid chromatography (HPLC). After successful validation of the chemometric model, the inline measurements were utilized for reaction characterisation. The acid-catalysed, second esterification step was identified as the limiting reaction step. From batch reactions conducted at different temperatures, the energy of activation of this step was determined to be 79.5 kJ mol−1. Additionally, kinetics were shown to follow a pseudo-first order with respect to the monoester formation and a kinetic model was established. The model was validated in simulations with changed reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...