Tuning radical interactions in trisradical tricationic complexes by varying host-cavity sizes†
Abstract
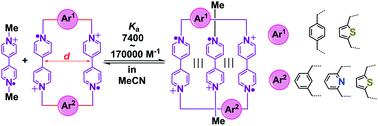
Although host–guest pairing interactions between bisradical dicationic cyclobis(paraquat-p-phenylene) (BB2(˙+)) and the bipyridinium radical cation (BIPY˙+) have been studied extensively, host molecules other than BB2(˙+) are few and far between. Herein, four bisradical dicationic cyclophanes with tunable cavity sizes are investigated as new bisradical dicationic hosts for accommodating the methyl viologen radical cation (MV˙+) to form trisradical tricationic complexes. The structure–property relationships between cavity sizes and binding affinities have been established by comprehensive solution and solid-state characterizations as well as DFT calculations. The association constants of the four new trisradical tricationic complexes are found to range between 7400 and 170 000 M−1, with the strongest one being 4.3 times higher than that for [MV⊂BB]3(˙+). The facile accessibility and tunable stability of these new trisradical tricationic complexes make them attractive redox-controlled recognition motifs for further use in supramolecular chemistry and mechanostereochemistry.


 Please wait while we load your content...
Please wait while we load your content...