Oxidative regulation of the mechanical strength of a C–S bond†
Abstract
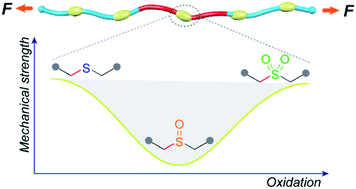
The mechanical strength of individual polymer chains is believed to underlie a number of performance metrics in bulk materials, including adhesion and fracture toughness. Methods by which the intrinsic molecular strength of the constituents of a given polymeric material might be switched are therefore potentially useful both for applications in which triggered property changes are desirable, and as tests of molecular theories for bulk behaviors. Here we report that the sequential oxidation of sulfide containing polyesters (PE-S) to the corresponding sulfoxide (PE-SO) and then sulfone (PE-SO2) first weakens (sulfoxide), and then enhances (sulfone), the effective mechanical integrity of the polymer backbone; PE-S ∼ PE-SO2 > PE-SO. The relative mechanical strength as a function of oxidation state is revealed through the use of gem-dichlorocyclopropane nonscissile mechanophores as an internal standard, and the observed order agrees well with the reported bond dissociation energies of C–S bonds in each species and with the results of CoGEF modeling.


 Please wait while we load your content...
Please wait while we load your content...