Iron-catalyzed remote functionalization of inert C(sp3)–H bonds of alkenes via 1,n-hydrogen-atom-transfer by C-centered radical relay†
Abstract
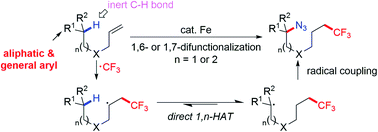
As an alternative approach to traditional C–H activation that often involved harsh conditions, and vicinal or primary C–H functionalization, radical relay offers a solution to these long-held problems. Enabled by 1,n (n = 5, 6)-hydrogen atom transfer (HAT), we use a most prevalent moiety, alkene, as the precursor to an sp3 C-centered radical to promote selective cleavage of inert C(sp3)–H bonds for the generation of azidotrifluoromethylated molecules. Mild conditions, broad scope and excellent regioselective control (>20 : 1) are observed in the reactions. Deuterium labelling studies disclose the kinetic characteristics of the transformations and verify a direct 1,n-HAT pathway. The key to this C-centered radical relay is that iron plays a dual role as a radical initiator and terminator to incorporate the azide functionality through radical oxidation via azido–ligand-transfer. The methods and the later derivatization promise expeditious synthesis of CF3-containing organic azides, γ-lactam and triazoles that are widely used in designing new fluorescent tags and functional materials.


 Please wait while we load your content...
Please wait while we load your content...