One pot synthesis of thio-glycosides via aziridine opening reactions†
Abstract
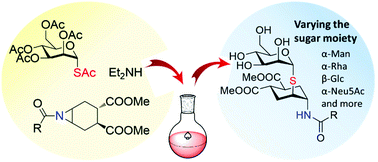
A one-pot aziridine opening reaction by glycosyl thiols generated in situ from the corresponding anomeric thio-acetates affords thio-glycosides with a pseudo-disaccharide structure and an N-linked tether. The scope of the one-pot aziridine opening reaction was explored on a series of mono- and disaccharides, creating a class of pseudo-glycosidic compounds with potential for further functionalization. Unexpected anomerization of glycosyl thiols was observed under the reaction conditions and the influence of temperature, base and solvent on the isomerization was investigated. Single isomers were obtained in good to acceptable yields for mannose, rhamnose and sialic acid derivatives. The class of thio-glycomimetics synthesized can potentially be recognized by various lectins, while presenting hydrolytic and enzymatic stability. The nitrogen functionality incorporated in the glycomimetics can be exploited for further functionalization, including tethering to linkers, scaffolds or peptide residues.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...