Enantioselective N-heterocyclic carbene-catalysed intermolecular crossed benzoin condensations: improved catalyst design and the role of in situ racemisation†
Abstract
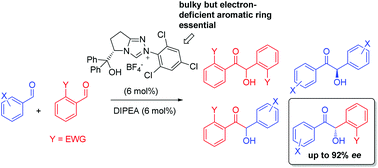
The enantioselective intermolecular crossed-benzoin condensation mediated by novel chiral N-heterocyclic carbenes derived from pyroglutamic acid has been investigated. A small library of chiral triazolium ions were synthesised. Each possessed a tertiary alcohol H-bond donor and a variable N-aryl substituent. It was found that increasing both the steric requirement and the electron-withdrawing characteristics of the N-aryl ring led to more chemoselective, efficient and enantioselective chemistry, however both quenching the reaction at different times and deuterium incorporation experiments involving the product revealed that this is complicated by product racemisation in situ (except in the case of benzoin itself), which explains the dependence of enantioselectivity on the electrophilicity of the reacting aldehydes common in the literature. Subsequent protocol optimisation, where one reacting partner was an o-substituted benzaldehyde, allowed a range of crossed-benzoins to be synthesised in moderate-good yields with moderate to excellent enantioselectivity.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...