Diastereodivergent synthesis of dispiroheterocyclic structures comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties†
Abstract
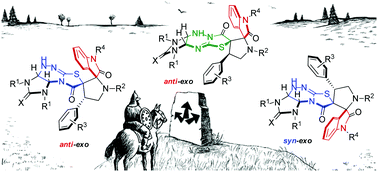
Highly diastereoselective methods for the synthesis of two different diastereomers of polynuclear dispiroheterocyclic compounds with five chiral centers comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties (dispiro[imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-7,3′-pyrrolidine-2′,3′′-indoles]) have been developed on the basis of a dipolar cycloaddition of azomethine ylides to benzylidene derivatives of imidazothiazolotriazines and an alkali-induced rearrangement of the thiazolotriazine fragment. The different sequence of the cycloaddition and rearrangement stages allows us to perform the targeted synthesis of two diastereomerically pure products from the same starting compounds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...