N-Primary-amine tetrapeptide-catalyzed highly asymmetric Michael addition of aliphatic aldehydes to maleimides†
Abstract
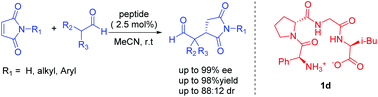
The highly asymmetric Michael addition reaction between maleimides and aliphatic aldehydes catalyzed by low-loading β-turn tetrapeptides with excellent yields and enantioselectivities at room temperature was reported. α-Branched and α-unbranched aldehydes both are suitable nucleophiles. N-Aryl, alkyl and hydrogen maleimides all are well tolerated and led to high yields and enantioselectivities. The transformation can be enlarged to the gram scale without decrease in the yield and enantioselectivity. Furthermore, the succinimides were converted into γ-lactams and γ-lactones, showing good practicality of this work. Some reaction intermediates in the proposed reaction mechanism can be captured with the HR-MS method.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...