Gold(i)–NHC-catalysed synthesis of benzofurans via migratory cyclization of 2-alkynylaryl ethers†
Abstract
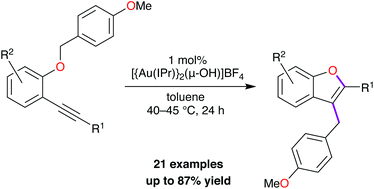
Homogeneous cationic gold(I) catalysis emerged as a preferred avenue for the activation of alkenes and alkynes towards reactions with weak nucleophiles, especially in cyclization reactions. Here we report an intramolecular carboalkoxylation reaction of electron-rich benzyl ethers of 2-ethynylaryl phenols catalysed by a digold(I)–NHC complex. The reaction proceeds efficiently with low catalyst loading and the resulting 2,3-disubstituted benzofurans form in moderate to good yields. Based on the results of a cross-over experiment, spectroscopic data, and DFT calculations, we propose a mechanism that accounts for the observed chemo- and regioselectivity.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...