A benzoxazole derivative as an inhibitor of anaerobic choline metabolism by human gut microbiota†
Abstract
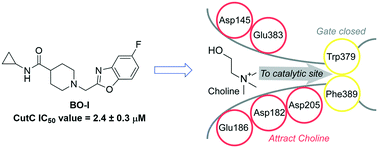
Metabolic pathways mediated by human gut bacteria have emerged as potential therapeutic targets because of their association with the pathophysiology of various human diseases. The anaerobic transformation of choline into trimethylamine (TMA) by gut microbiota is directly linked to type 2 diabetes, fatty liver disease, and cardiovascular diseases. Structural analogs of choline have been developed as competitive inhibitors of choline TMA-lyase (CutC), a key enzyme for the conversion of choline to TMA. However, weak to moderate CutC inhibitory profiles of the choline analogs limit their further advancement into clinical translation. In this study, we introduce a glycomimetic-based approach for the identification of CutC inhibitors with intestinal metabolic stability. Our workflow started with screening of a small library of glycomimetics for metabolic stability in the presence of human intestinal S9 fraction. Further screening using an in vitro CutC inhibitory assay identified a benzoxazole ligand (BO-I) as a CutC inhibitor with an IC50 value of 2.4 ± 0.3 μM. Kinetic analysis revealed that BO-I functions as a non-competitive inhibitor of CutC. Interestingly, BO-I reduced the production of TMA in whole cell assays of multiple bacterial strains as well as in complex biological environments. Therefore, structural optimization of BO-I holds promise for the development of efficient gut microbiota-targeted small molecules.


 Please wait while we load your content...
Please wait while we load your content...