Plasma–liquid synthesis of MoOx and WO3 as potential photocatalysts†
Abstract
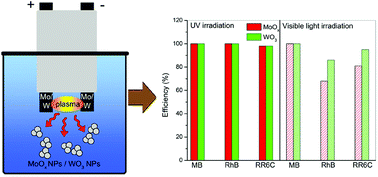
Plasmas in contact with liquids represent a green chemistry method for the synthesis of metal oxides. In this work, underwater plasma was used for the synthesis of molybdenum and tungsten oxides. The obtained samples were analyzed by various techniques. Results showed that underwater plasma with Mo electrodes allows obtaining non-stoichiometric molybdenum oxide (MoOx). In the case of tungsten electrodes, monoclinic WO3 was formed. The synthesized oxides have a wide band gap (3.21 eV for MoOx and 3.27 eV for WO3). The photocatalytic and sorption activities of the synthesized oxides towards the decomposition of cationic and anionic dyes (Methylene Blue, Rhodamine B, and Reactive Red 6C) were studied. MoOx shows excellent photocatalytic performance under UV and visible light irradiation. The photocatalytic activity of WO3 under visible light is less than that under UV irradiation.


 Please wait while we load your content...
Please wait while we load your content...