Synthesis, structural characterization and electrochemical and magnetic studies of M(hfac)2 (M = CuII, CoII) and Nd(hfac)3 complexes of 4-amino-TEMPO†
Abstract
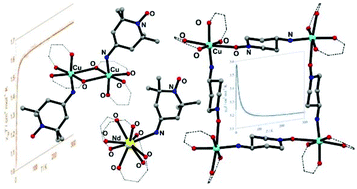
Three mononuclear complexes [M(hfac)x(ATEMPO)y], where M = Cu (11) and Co (12), x = y = 2; M = Nd (13), x = 4, y = 1, and two polynuclear complexes [{Cu(hfac)2(ATEMPO)}n], where n = 2 (14) and 4 (15), were obtained by the reaction of M(hfac)x (M = CuII, CoII, NdIII; x = 2, 3) with 4-amino-TEMPO (4-amino-2,2,6,6-tetramethylpiperidin-N-oxyl) in good yields and their structural, electrochemical and magnetic properties were examined. In all cases, the radical is coordinated to the metal through the amino group, except 15, and the metal ions have an octahedral geometry, except 13. Different coordination architectures of the copper complexes were obtained as a function of the stoichiometry and solvents used. In complexes 11 and 12 the radicals show an equatorial–equatorial and axial–equatorial arrangement, respectively, giving rise to two distinct 2D supramolecular systems through intermolecular interactions. Compound 13 is the first example of a lanthanide complex of the ATEMPO radical. The NdIII ion adopts a rare nine-coordination via binding to four hfac ligands and the radical. The dinuclear complex 14 shows a (Cu–O)2 core in which the CuII ions are bridged by the oxygen atoms from the hfac ligands. In compound 15 the ATEMPO radical acts as a bidentate ligand through the amino and nitroxyl groups leading to an unprecedented tetranuclear square-shaped framework. Cyclic voltammetry showed redox processes associated with the copper and TEMPO moieties. Electrochemical impedance spectroscopy revealed the temperature dependence of the conductivity for compound 15 with a maximum of 2.09 × 10−5 S cm−1 at 408 K. The magnetic behavior of complexes 11–15 is determined by metal–radical interactions. Ferromagnetic interaction has been observed for complex 11 due to the existence of two different exchange pathways arising from the conformational arrangement of the radicals around the metal center, whereas the single conformation of the radical in complex 14 resulted in a weak antiferromagnetic coupling. In complex 15 both O–Cu and N–Cu contacts are present giving rise to ferromagnetic and antiferromagnetic interactions, respectively.


 Please wait while we load your content...
Please wait while we load your content...