Second order Jahn–Teller interactions at unusually high molecular orbital energy separations†
Abstract
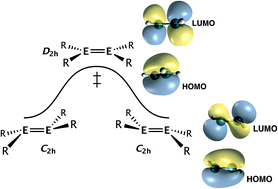
Second order Jahn–Teller (SOJT) effects arise from interactions between filled and empty molecular orbitals of like symmetry. These interactions often lead to structural distortions whose extent is inversely proportional to the energy difference between the interacting orbitals. The main objectives of the work described here are (1) the calculation (using density functional theory methods) of the energies of the valence molecular orbitals in the species EH3 (E = N, P, As or Sb), HEEH (E = C, Si, Ge or Sn), and H2EEH2, (E = C, Si, Ge or Sn) and (2) the correlation of these energies with barriers for planarization or linearization. The calculations suggest an upper limit of about 12 eV energy separation of the interacting levels for SOJT effects to be significant, which is considerably larger than previously thought and implies that SOJT effects may be more common than currently realized.


 Please wait while we load your content...
Please wait while we load your content...