Structural characteristics of oligomers formed by pyroglutamate-modified amyloid β peptides studied by solid-state NMR†
Abstract
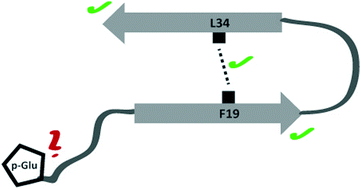
Neuronal plaques of amyloid β (Aβ) peptides of varying length carrying different posttranslational modifications represent a molecular hallmark of Alzheimer's disease. It is believed that transient oligomeric Aβ assemblies associating in early fibrillation events represent particularly cytotoxic peptide aggregates. Also, N-terminally truncated (in position 3 or 11) and pyroglutamate modified peptides exhibited an increased toxicity compared to the wildtype. In the current study, the molecular structure of oligomeric species of pGlu3-Aβ(3–40) and pGlu11-Aβ(11–40) was investigated using solid-state NMR spectroscopy. On the secondary structure level, for both modified peptides a large similarity between oligomers and mature fibrils of the modified peptides was found mainly based on 13C NMR chemical shift data. Some smaller structural differences were detected in the vicinity of the respective modification site. Also, the crucial early folding molecular contact between residues Phe19 and Leu34 could be observed for the oligomers of both modified peptide species. Therefore, it has to be concluded that the major secondary structure elements of Aβ are already present in oligomers of pGlu3-Aβ(3–40) and pGlu11-Aβ(11–40). These posttranslationally modified peptides arrange in a similar fashion as observed for wild type Aβ(1–40).


 Please wait while we load your content...
Please wait while we load your content...