Pyroelectrically-driven chemical reactions described by a novel thermodynamic cycle†
Abstract
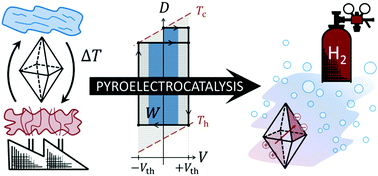
Pyroelectrocatalysis is the conversion of thermal energy directly into chemical energy. On the background of renewable energies and the need for efficient industrial processes, the conversion of waste heat into hydrogen is of special relevance. Since the reported thermodynamic cycles for pyroelectric energy harvesting do not fit the conditions encountered in a reactive medium such as water appropriately, we describe a new thermodynamic charge-voltage-cycle characterised by fixed upper and lower potentials. These threshold potentials comprise the redox potential of the reaction of interest – here the hydrogen evolution reaction – as well as an overpotential mainly dictated by the temperature-induced bending of electronic bands in the pyroelectric semiconductor. Because polarisation changes below the threshold are useless for chemical reactions, material properties as well as process conditions have to be chosen accordingly. In particular the particle size along with the temperature difference are shown to determine the conversion efficiency.


 Please wait while we load your content...
Please wait while we load your content...