About the origin of the large Stokes shift in aminoalkyl substituted heptamethine cyanine dyes†
Abstract
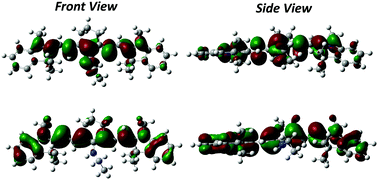
Aminoalkyl-substituted heptamethine cyanine dyes are characterized by a large Stokes shift, an uncommon feature for cyanine molecules yet very promising for their application as fluorescent probes in bioimaging and as light harvesting antennas in biohybrid systems for solar energy conversion. The origin of this photophysical feature has not been adequately explored so far, and a combined experimental and theoretical work is herein provided to shed light on the role played by the central aminoalkyl substituent bound to the heptamethine cyanine backbone in defining the unusual properties of the dye. The spectra recorded in solvents of different polarities point to a marginal role of the medium in the definition of the Stokes shift, which conversely can be ascribed to the relaxation of the molecular geometry upon photoexcitation. This hypothesis is supported by an extensive theoretical investigation of the ground and excited states of the dye. TD-DFT results on the aminoalkyl-substituted dye and its unsubstituted precursor demonstrate a very similar cyanine-like structure for both molecules in the relaxed excited state. Conversely, in the ground state the amino substitution disrupts the conjugation in the polymethine chain, leading to a broken-symmetry, non-planar structure.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...