Copper-catalysed ortho-selective C–H bond functionalization of phenols and naphthols with α-aryl-α-diazoesters†
Abstract
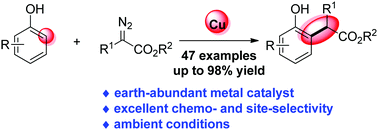
Herein, we reported the first copper-catalyzed highly efficient C(sp2)–H functionalization of unprotected naphthols and phenols with α-aryl-α-diazoesters. In this transformation, CuCl2 efficiently promoted the highly chemo- and site-selective C–H bond functionalization under mild conditions, furnishing diverse phenol derivatives in moderate to excellent yields from readily available starting materials.
- This article is part of the themed collection: Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...