Terminal-oxidant-free photocatalytic C–H alkylations of heteroarenes with alkylsilicates as alkyl radical precursors†
Abstract
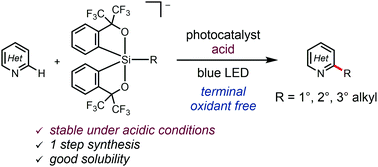
We report the photocatalytic C–H alkylations of heteroarenes with alkylsilicates bearing C,O-bidentate ligands under acidic conditions. Irradiation of heteroaromatics in the presence of the silicates and trifluoroacetic acid produced the corresponding alkylated compounds. The present reaction system does not require any terminal oxidant although the reaction seems to be a formal oxidation reaction. This study demonstrates that alkylsilicates can be used in photocatalytic radical chemistry under acidic conditions.


 Please wait while we load your content...
Please wait while we load your content...