Nickel(ii)-catalyzed asymmetric thio-Claisen rearrangement of α-diazo pyrazoleamides with thioindoles†
Abstract
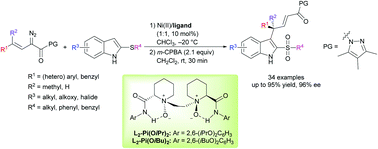
A nickel(II) catalyzed enantioselective thio-Claisen rearrangement of α-diazo pyrazoleamides with thioindoles was realized with modified chiral N,N′-dioxide ligands, affording a variety of C3-substituted indole derivatives in high yields (up to 95%) with excellent enantioselectivities (up to 96% ee) under mild reaction conditions. A possible transition state model was proposed based on previous reports and the X-ray crystal structure of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...