Hydride, chloride, and bromide show similar electronic effects in the Au9(PPh3)83+ nanocluster†
Abstract
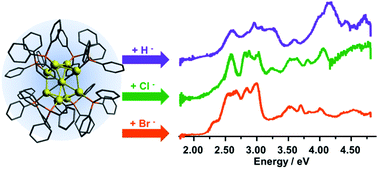
Recent experimental and computational results have suggested that a hydride bound to the Au9(PPh3)83+ gold nanocluster donates its electrons to the metal core, forming an 8-electron cluster. We present electronic absorption spectra of precisely mass-selected Au9(PPh3)83+ clusters featuring a surface hydride, chloride, or bromide. Comparison of these spectra shows that H−, Cl−, or Br− perturb the electronic structure of the Au9(PPh3)83+ core in similar ways. This suggests that hydride and halides play similar roles electronically in this cluster, and thus are either both metallic or both ligand-like.


 Please wait while we load your content...
Please wait while we load your content...