Indium controlled regioselective 1,4-alkylarylation of 1,3-dienes with α-carbonyl alkyl bromides and N-heterocycles†
Abstract
We here describe a new, selective indium-promoted silver-mediated intermolecular oxidative 1,4-alkylarylation of 1,3-dienes with α-carbonyl alkyl bromides and N-heterocycles for producing functionalized N-heterocycles, which is characterized by its exquisitely controllable regio-/stereo-selectivity and excellent tolerance of functional groups. Mechanistically, the formation of the carbonyl-coordinated η3-allyl-In complex radical intermediate is the key factor for successfully achieving regio- and stereo-selectivity toward 1,4-difunctionalization and (E)-isomers.
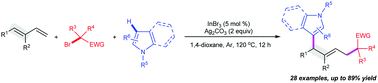


 Please wait while we load your content...
Please wait while we load your content...