Improved charge transport in fused-ring bridged hemi-isoindigo-based small molecules by incorporating a thiophene unit for solution-processed organic field-effect transistors†
Abstract
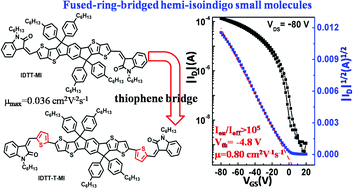
Two acceptor–donor–acceptor small molecules based on a fused ring indacenodithieno[3,2-b]thiophene (IDTT) as a donor, and hemi-isoindigo units, methyleneoxindole (IDTT-MI) and thienylmethyleneoxindole (IDTT-T-MI) as acceptors were synthesized and characterized for application in solution-processed organic field-effect transistors. The incorporation of a thiophene bridge which extended the conjugation of the backbone maintained highly co-planar structures, and also endowed the small molecule with ordered crystalline structures and interconnected morphology. Consequently, IDTT-T-MI-based organic field-effect transistors exhibited a highest hole mobility of 0.80 cm2 V−1 s−1, which was the highest mobility for solution-processed hemi-isoindigo-based organic semiconductor materials.


 Please wait while we load your content...
Please wait while we load your content...