Dual phosphorescence from the organic and inorganic moieties of 1D hybrid perovskites of the Pbn′Br4n′+2 series (n′ = 2, 3, 4, 5)†
Abstract
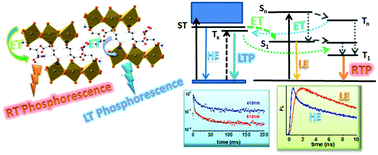
The diprotonated lysine – 2,6-diamino-hexanoic acid – (H2Lys2+) and ornithine – 2,5-diamino-pentanoic acid – (H2Orn2+) dications in the presence of lead bromide afford four novel hybrid perovskites: (DL-H2Lys)3[Pb2Br10]·3H2O (1), (L-H2Orn)4[Pb3Br14]·2H2O (2), (L-H2Lys)6[Pb4Br18]·2Br·2H2O (3) and (DL-H2Lys)6[Pb5Br22]·4H2O (4). In all structures 1–4, the inorganic frameworks are 1D perovskite ribbons belonging to the Pbn′Br4n′+2 series, n′ corresponding to the number of octahedra of the ribbon width. The n′ = 3, n′ = 4 and n′ = 5 networks enlarge the very rare cases of such perovskite anions. The crystallized powders of all the compounds display bright orange broad-band emission with efficiencies up to 28% and room temperature phosphorescence in the ms range promoted by energy transfer processes from the inorganic to the organic moieties. In the films the lower efficiency of energy transfer enhances the relative intensity of the blue emission component, providing white light emission at room temperature. At 84 K additional narrow blue phosphorescence from the inorganic moieties, with a lifetime of 19 ms, provides unprecedent dual phosphorescence emission.


 Please wait while we load your content...
Please wait while we load your content...