Efficient triphenylamine-based polymorphs with different mechanochromism and lasing emission: manipulating molecular packing and intermolecular interactions†
Abstract
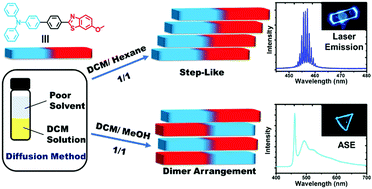
Organic polymorphs with tunable luminescence are crucial in developing organic luminescent materials, but the regulation of molecular packing modes and intermolecular interactions in organic crystals remains a challenge. Here, we report three triphenylamine–benzothiazole (TZ) compounds by systematically changing the substituents, resulting in different crystal emission characteristics. The polymorphs of blue emission (TZ-1B) and cyan emission (TZ-1C) crystals are obtained by controlling the crystallization conditions, however, we could not obtain organic polymorphs of TZ-2 or TZ-3 in various solvent systems. By molecular systems and detailed single crystal analysis, it is found that appropriate substituents play a key role in manipulating the intermolecular interactions and the molecular packing modes to affect the optical properties of organic crystals. Moreover, TZ-1C exhibits blue-shifted mechanochromism, while TZ-1B does not. More importantly, TZ-1B exhibits a lasing emission at 454 nm with a low threshold and a high cavity quality factor. TZ-1C exhibits amplified spontaneous emission (ASE) at 462 nm. Thus, the molecular systems provide a reasonably potent molecular strategy to understand the molecular packing structure–fluorescence property relationship.


 Please wait while we load your content...
Please wait while we load your content...