Screening of pH-responsive long-circulating polysaccharide–drug conjugate nanocarriers for antitumor applications†
Abstract
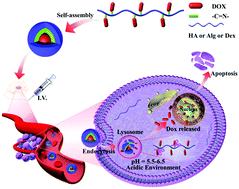
For the treatment of malignant tumors, drug nanocarriers with long blood circulation time and ability to target the tumor microenvironment are promising therapeutic abilities. In this work, to systematically investigate the roles and functions of polysaccharides as drug nanocarriers targeting the tumor microenvironment, different types of polysaccharides (alginic acid (Alg), hyaluronic acid (HA), and dextran (Dex)) were covalently bonded with doxorubicin (DOX) through a Schiff base reaction to form a pH-sensitive polysaccharide–DOX prodrug having an acid-sensitive imine bond. After screening, Dex-DOX exhibited high drug loading content and good stability, while Alg-DOX and HA-DOX may have disadvantages such as low degree of oxidation, limited drug loading capacity, or instability in physiological conditions. Dex-DOX prodrugs were able to self-assemble into stable nanoparticles in phosphate buffered saline (PBS). Then, Dex6k-DOX and Dex150k-DOX were selected for further comparisons since they had similar drug-binding rates and long circulation time. When compared with Dex6k-DOX, the longer main-chain Dex150k-DOX showed a higher drug release rate under simulated acidic conditions in vitro, which significantly inhibited cell proliferation. Further in vivo experiments showed that Dex150k-DOX could more effectively improve the antitumor efficiency and survival rate while reducing side-effects. Overall, the screening and comparisons provided detailed and systematical information about the polysaccharide–DOX prodrug platform as potential antitumor drugs.


 Please wait while we load your content...
Please wait while we load your content...