Acid-breakable TPGS-functionalized and diallyl disulfide-crosslinked nanogels for enhanced inhibition of MCF-7/ADR solid tumours†
Abstract
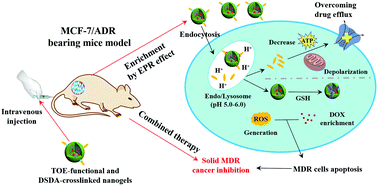
Herein, a diallyl disulfide (DADS)-crosslinked nanogel (NG) and an ortho ester-conjugated TPGS (T-OE) were synthesized using free radical copolymerization and transesterification, respectively. Then, T-OE was grafted onto the NG to fabricate a dual-functionalized nanogel (TNG), and both the NG and TNG possessed a uniform diameter (∼160 nm) and excellent stability. The DOX-loaded nanogel (NG/D and TNG/D) displayed appropriate release properties under reducing conditions. MCF-7/ADR cell experiments showed that although both the NG/D and TNG/D could increase the production of reactive oxygen species (ROS), only the TNG could effectively overcome the drug efflux by inducing mitochondrial depolarization and by interfering with the metabolism of ATP. Both the cell cytotoxicity and the MCF-7/ADR solid cancer assay indicated that TNG/D possessed a long-acting drug enrichment and enhanced the inhibition, resulting from the combined action of the high ROS level and the suppressed drug efflux. These results demonstrated that the T-OE-functionalized and the DADS-crosslinked NG had a great potential for use in the treatment of MCF-7/ADR tumours.


 Please wait while we load your content...
Please wait while we load your content...