Depth-dependent oxygen redox activity in lithium-rich layered oxide cathodes†
Abstract
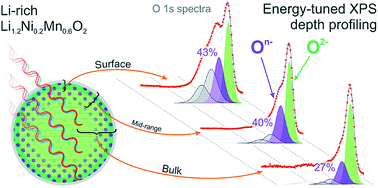
Lithium-rich materials, such as Li1.2Ni0.2Mn0.6O2, exhibit capacities not limited by transition metal redox, through the reversible oxidation of oxide anions. Here we offer detailed insight into the degree of oxygen redox as a function of depth within the material as it is charged and cycled. Energy-tuned photoelectron spectroscopy is used as a powerful, yet highly sensitive technique to probe electronic states of oxygen and transition metals from the top few nanometers at the near-surface through to the bulk of the particles. Two discrete oxygen species are identified, On− and O2−, where n < 2, confirming our previous model that oxidation generates localised hole states on O upon charging. This is in contrast to the oxygen redox inactive high voltage spinel LiNi0.5Mn1.5O4, for which no On− species is detected. The depth profile results demonstrate a concentration gradient exists for On− from the surface through to the bulk, indicating a preferential surface oxidation of the layered oxide particles. This is highly consistent with the already well-established core–shell model for such materials. Ab initio calculations reaffirm the electronic structure differences observed experimentally between the surface and bulk, while modelling of delithiated structures shows good agreement between experimental and calculated binding energies for On−.


 Please wait while we load your content...
Please wait while we load your content...