Micropore-confined amorphous SnO2 subnanoclusters as robust anode materials for Na-ion capacitors†
Abstract
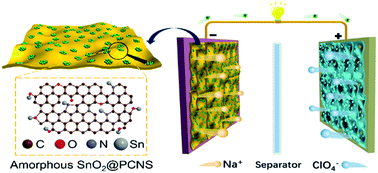
Electrode materials with amorphous and subnano structures commonly exhibit excellent Na-ion storage performance. However, the high surface specific energy of such materials spontaneously causes thermodynamic agglomeration and recrystallization, limiting their underlying capability in energy storage applications. For the first time, we realize the synthesis of amorphous SnO2 (A-SnO2) subnanoclusters (with an average size of ∼0.93 nm) by a facile and controllable strategy using N, O-doped porous carbon nanosheets as the support material. The A-SnO2 subnanoclusters with stretched Sn–O and Sn–Sn bonds can stably exist because of the microporous confinement and strong interfacial bonding afforded by the support. The A-SnO2 subnanocluster anode shows a higher reversible capacity and better rate capability than the large-sized crystalline SnO2 nanoparticle anode fabricated using graphene oxide as the alternative support. Electrochemical characterization and first-principles calculations show that the synergistic effect of unique SnO2 and the superior carbon support enhance the storage capacity and diffusion coefficient of Na-ions. Moreover, particle pulverization and exfoliation of the SnO2 anode is obviously suppressed during desodiation/sodiation, leading to a superior cycling performance (without capacity decay after 1400 cycles). Consequently, a Na-ion capacitor assembled using the A-SnO2 subnanocluster anode exhibits a high energy (196.4 W h kg−1), high power (28.1 kW kg−1), and long lifetime.


 Please wait while we load your content...
Please wait while we load your content...