Oxygen defect engineering in cobalt iron oxide nanosheets for promoted overall water splitting†
Abstract
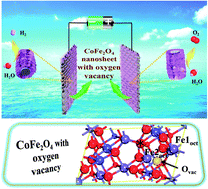
Transition metal oxides have attracted tremendous attention as active and stable electrocatalysts for hydrogen or oxygen evolution from water splitting. However, their application as bifunctional catalysts for overall water splitting is still hindered by their limited activity. In this paper, via the surface defect engineering strategy, a bifunctional electrocatalyst based on oxygen vacancy enriched CoFe2O4 (r-CFO) nanosheets was successfully fabricated, exhibiting desired overall water splitting activity. DFT calculations demonstrated that benefitting from the incorporation of oxygen vacancies, the adsorption energy (Eads) of H2O and the Gibbs free energy change for hydrogen adsorption (ΔGH*) are both well optimized, leading to the fine modulation of active site activity. Meanwhile, along with oxygen vacancy doping, the density of states across the Fermi level increased as well, which would be conducive to fast electron transportation. As expected, the r-CFO catalyst afforded obviously lower overpotentials of 280 mV and 121 mV to achieve a current density of 10 mA cm−2 for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), respectively. Furthermore, r-CFO exhibited excellent overall water splitting activity with a voltage of 1.53 V to reach a current density of 10 mA cm−2. This work highlights the vital role of surface defect engineering based on transition metal oxides toward advanced electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...