Regulating exciton bonding energy and bulk heterojunction morphology in organic solar cells via methyl-functionalized non-fullerene acceptors†
Abstract
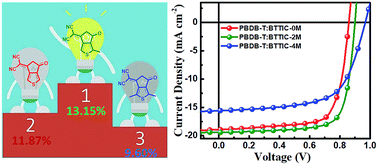
Electron-deficient end groups (EGs) are very important for non-fullerene small molecule acceptors (NF-SMAs) to tune their absorption, energy levels, and crystallization properties. Herein, we designed and synthesized three SMAs, namely, BTTIC-0M, BTTIC-2M, and BTTIC-4M by adding the methyl unit into 2-(6-oxo-5,6-dihydro-4H-cyclopenta[c]thiophen-4-ylidene)malononitrile (CPTCN). Methyl group, with its slight electron-donating ability, significantly elevates the LUMO energy levels but does not seriously affect the bandgaps of the CPTCN-based SMAs, which helps to reduce the energy loss (Eloss). In-depth dynamic theoretical simulations of the donor–acceptor (D–A) complex reveal that the exciton bonding energy (BE) can be fine-tuned by continuously increasing the methyl groups on the end groups of the SMAs. Methyl-substituted EG reduces the driving force and also enhances the BE of the charge transport (CT) state exciton, leading to a decrease in the exciton dissociation efficiencies. However, we found that one methyl-functionalized CPTCN enables PBDB-T:BTTIC-2M-based organic solar cells (OSCs) to achieve a power conversion efficiency (PCE) as high as 13.15%. Though PBDB-T:BTTIC-2M-based OSCs exhibit a slightly lower exciton dissociation efficiency than those of PBDB-T:BTTIC-0M, a more favorable superficial and internal morphology is attained in the PBDB-T:BTTIC-2M bulk-heterojunction layer, which balances the electron and hole mobilities and diminishes the bimolecular recombination. Comparatively, BTTIC-4M failed to realize a high performance owing to its adverse interactions with the polymer chain and the multiscale phase separation in the blend films. Actually, adjusting the number of methyl groups on the end group is done to compensate the current–voltage losses within the OSC devices with complicated contributions from absorption spectra, LUMO energy levels, exciton bonding energies, and morphologies.


 Please wait while we load your content...
Please wait while we load your content...