Band vs. polaron: vibrational motion and chemical expansion of hydride ions as signatures for the electronic character in oxyhydride barium titanate
Abstract
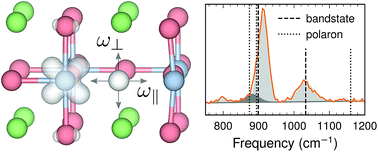
The oxyhydride phase of barium titanate, BaTiO3−xHx, is a mixed hydride ion and electron conductor. The substitution of oxygen with hydrogen to form a hydride ion is accompanied by donation of an electron to the initially empty titanium 3d conduction band. It is not clear, however, whether the electron forms a delocalized state where it is shared among all titanium ions forming a bandstate, or if it localizes on a titanium ion and forms a bound electron polaron. Here, we investigate polaron formation in this material using density-functional theory (DFT) calculations, where the self-interaction error has been corrected by the DFT + U method and the HSE hybrid functional. While calculated formation energies do not provide a conclusive description of the electronic state, a comparison of the results from first-principles phonon calculations with vibrational spectra measured with inelastic neutron scattering (INS) suggests that the electrons form bandstates in bulk BaTiO3−xHx. This is further supported by comparison of the computed chemical expansion of the involved defect species with experimental data of the lattice expansion in the oxyhydride formation. The oxyhydride phase of barium titanate, BaTiO3−xHx, should thus exhibit metallic-like conductivity.


 Please wait while we load your content...
Please wait while we load your content...