Atomistic understanding of structural evolution, ion transport and oxygen stability in layered NaFeO2†
Abstract
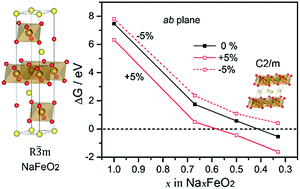
α-NaFeO2 shares a structure similar to many layered electrode materials in Li-ion and Na-ion batteries. In this work, first-principles calculations are carried out to gain atomistic understanding of structural evolution, ion transport and oxygen stability in NaFeO2. Based on the calculation results, we provide an atomistic description of phase transition and structural changes during the charging process. Meanwhile, we identify a di-vacancy assisted diffusion mechanism for Na ions and estimate the diffusion barrier that agrees with experimental data. Furthermore, we reveal that lattice strains could modulate both ion transport and oxygen stability in NaFeO2. A moderate 3% tension in the out-of-plane direction could render the ion diffusion barrierless. Moreover, it is predicted that in-plane compressions can stabilize oxygen and suppress oxygen evolution at high potentials. Thus, a combination of the out-of-plane tension with the in-plane compression is expected to reduce the diffusion barrier and stabilize oxygen simultaneously.


 Please wait while we load your content...
Please wait while we load your content...