Reversible oxygen intercalation in hexagonal Y0.7Tb0.3MnO3+δ: toward oxygen production by temperature-swing absorption in air†
Abstract
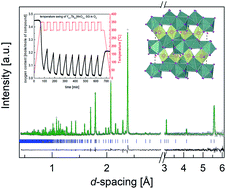
The oxygen storage capacity, structure and thermodynamic stability were studied for hexagonal Y0.7Tb0.3MnO3+δ in oxygen and air to assess its applicability for oxygen separation from air by a temperature-swing adsorption process. We show that large amounts of oxygen excess can be reversibly incorporated into and extracted from small particle-size samples of Y0.7Tb0.3MnO3+δ prepared by sol–gel synthesis for fixed oxygen partial pressures. The hyperstoichiometric material, with δ ≥ 0.45 prepared in oxygen or high-pressure oxygen atmospheres, assumes a new hexagonal structure with interstitial oxygen defects near the nominally five coordinated Mn site as determined by neutron diffraction and supported by scanning and transmission electron microscopy. Thermogravimetric measurements demonstrate reversible intercalation of oxygen in a pure O2 atmosphere at around 300 °C, but more importantly, also in air over a remarkably narrow temperature range of ∼20 °C, albeit producing smaller oxygen amounts δ ≤ 0.25. Comparison of samples' properties obtained by the sol–gel and solid-state synthesis methods confirms enhanced oxygen storage capacity and oxygen exchange kinetics for the small particle-size samples which exhibit larger specific surface areas. Sequential temperature-swing absorption and long-term annealing experiments demonstrate the high practical potential of Y0.7Tb0.3MnO3 compounds for the industrial production of oxygen enriched gases by utilization of waste heat at 250–350 °C.


 Please wait while we load your content...
Please wait while we load your content...