A thermal energy storage prototype using sodium magnesium hydride†
Abstract
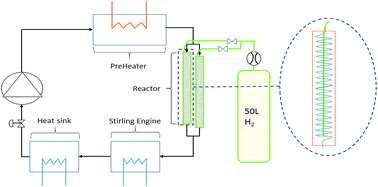
Metal hydrides present favourable thermal storage properties particularly due to their high energy density during thermochemical hydrogenation. For this purpose, sodium magnesium hydride (NaMgH3) has shown promising qualities that could lead to an industrialised application, but first requires to be examined on a lab-scale under realistic operating conditions. Herein, the cycling reversibility of NaMgH3 is undertaken on a 150 g scale with active heat extraction and delivery using superheated water vapour as the heat transfer fluid. The thermal and cycling properties of the hydride material are enhanced by addition of TiB2 and exfoliated natural graphite. Over 40 cycles the NaMgH3 showed minimal loss in capacity, but revealed difficulties in terms of thermal management to avoid local overheating, resulting in the production of undesired molten sodium metal. The temperature cycling showed a hydrogen flow culminating at 1 g h−1, which was insufficient to ensure thermal energy retrieval. The increase of the inlet hydrogen pressure has been shown to be instrumental in achieving an acceptable flow rate of 10 g h−1. Indeed, this design, despite high heat losses to the environment, was able to supply a third of the chemical energy available to the heat transfer fluid.


 Please wait while we load your content...
Please wait while we load your content...