Cobalt-based ferrites as efficient redox materials for thermochemical two-step CO2-splitting: enhanced performance due to cation diffusion†
Abstract
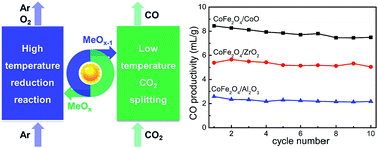
Thermochemical two-step CO2 splitting under concentrated solar energy has received significant attention, and ferrites are promising materials for such a redox reaction. However, their application is still challenged by the sintering problem due to the inevitably high operating temperature. Herein, we report non-stoichiometric cobalt-based ferrites, with no thermostable supports employed, as efficient redox materials for thermochemical two-step CO2 splitting. In comparison with ZrO2- and Al2O3-added CoFe2O4, CoO-added CoFe2O4 (CoFe2O4/CoO) shows a CO productivity of 8.5 mL g−1, which is 1.5 and 3.2 times higher than that of CoFe2O4/ZrO2 (5.4 mL g−1) and CoFe2O4/Al2O3 (2.6 mL g−1), respectively. Based on the morphological and crystal structure characterization by X-ray diffraction (XRD), Raman spectroscopy, scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), Mössbauer spectroscopy and Fourier transform infrared spectroscopy (FT-IR), the ferrites were thoroughly analyzed and thereby bulk cation diffusion for CoFe2O4/CoO over the reaction is kinetically demonstrated to play an important role in enhancing the performance.


 Please wait while we load your content...
Please wait while we load your content...