Hydrocarbon-soluble, hexaanionic fulleride complexes of magnesium†
Abstract
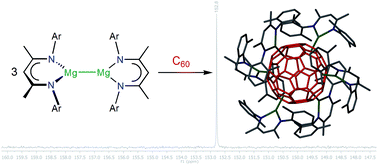
The reaction of the magnesium(I) complexes [{(Arnacnac)Mg}2], (Arnacnac = HC(MeCNAr)2, Ar = Dip (2,6-iPr2C6H3), Dep (2,6-Et2C6H3), Mes (2,4,6-Me3C6H2), Xyl (2,6-Me2C6H3)) with fullerene C60 afforded a series of hydrocarbon-soluble fulleride complexes [{(Arnacnac)Mg}nC60], predominantly with n = 6, 4 and 2. 13C{1H} NMR spectroscopic studies show both similarities (n = 6) and differences (n = 4, 2) to previously characterised examples of fulleride complexes and materials with electropositive metal ions. The molecular structures of [{(Arnacnac)Mg}nC60] with n = 6, 4 and 2 can be described as inverse coordination complexes of n [(Arnacnac)Mg]+ ions with C60n− anions showing predominantly ionic metal–ligand interactions, and include the first well-defined and soluble complexes of the C606− ion. Experimental studies show the flexible ionic nature of the {(Arnacnac)Mg}+⋯C606− coordination bonds. DFT calculations on the model complex [{(Menacnac)Mg}6C60] (Menacnac = HC(MeCNMe)2) support the formulation as an ionic complex with a central C606− anion and comparable frontier orbitals to C606− with a small HOMO–LUMO gap. The reduction of C60 to its hexaanion gives an indication about the reducing strength of dimagnesium(I) complexes.


 Please wait while we load your content...
Please wait while we load your content...