The debut of chiral cyclic (alkyl)(amino)carbenes (CAACs) in enantioselective catalysis†
Abstract
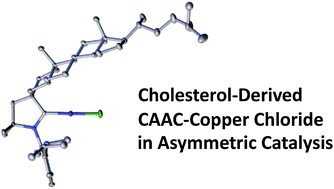
The popularity of NHCs in transition metal catalysis has prompted the development of chiral versions as electron-rich neutral stereodirecting ancillary ligands for enantioselective transformations. Herein we demonstrate that cyclic (alkyl)(amino)carbene (CAAC) ligands can also engage in asymmetric transformations, thereby expanding the toolbox of available chiral carbenes.


 Please wait while we load your content...
Please wait while we load your content...