Nucleophile-intercepted Beckmann fragmentation reactions†
Abstract
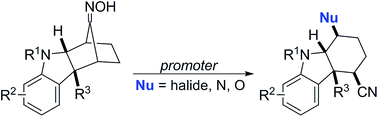
We describe the first examples of nucleophile-intercepted Beckmann fragmentations of indoline oximes. This reaction uses MsCl as a promoter to give cyano chlorides and is believed to proceed through an aziridinium intermediate via a double stereoinvertive process. Mechanistic insights have led to the further discovery that oxygen, nitrogen, and bromide nucleophiles can be employed for this fragmentation by the use of other promoters. We envision that these products may be useful in the syntheses of members of the akuammiline and koumine families of indoline alkaloids.


 Please wait while we load your content...
Please wait while we load your content...